NEUROLOGY
Use Recommendations for Aducanumab in Alzheimer Disease
Research Review and Resources
Sign up for Figure 1 and be notified directly of new clinical cases and related research, CME activities, quizzes, news, and trends. It’s free!

Figure 1, 2, 3

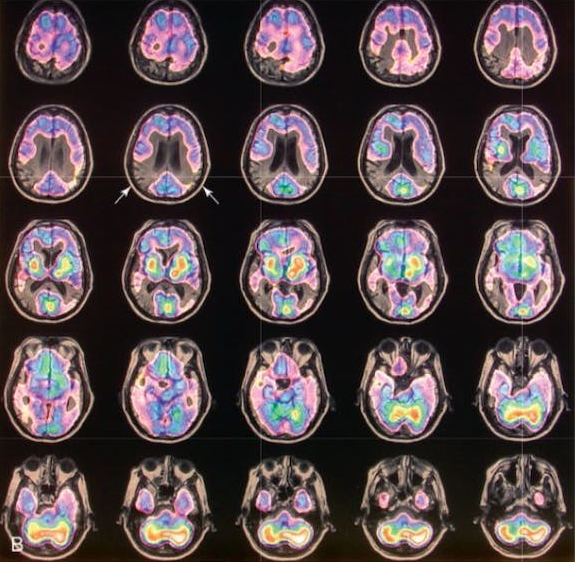
1. Appropriate patients have mild cognitive impairment or mild Alzheimer disease with cerebral amyloid deposition confirmed by PET scan or CSF analysis.

2. The greatest risk is amyloid-related imaging abnormalities, with or without cerebral edema and hemorrhage. Contraindications include coagulopathy and prior brain bleeds/microhemorrhages.

3. Patients should be counseled that the expected outcome is possible slowing of disease progression, not improvement in symptoms or daily function.
Summary
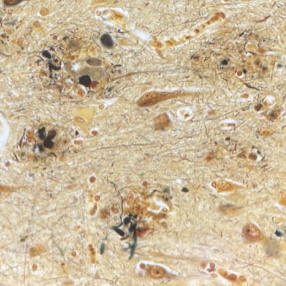
Aducanumab is a first-in-class anti-amyloid recombinant human immunoglobulin G1 (IgG1) monoclonal antibody. It was designed to selectively promote clearance of insoluble forms of A𝛽 and cerebral amyloid aggregates (“amyloid plaques”) which are believed to play a key role in Alzheimer disease pathology.
Interested patients should be aware that aducanumab will not reverse current symptoms. It clears cerebral amyloid by objective measures, which theoretically should slow or stop the disease process. However, there is currently no evidence of clinical benefit in terms of improved daily function or symptom reduction as a result of this clearance. Aducanumab may slow disease progression by an unclear amount. Additionally, patients should be informed of treatment burdens such as monthly infusions, frequent monitoring including repeated imaging studies if needed, and high cost. Medicare, alongside many private insurance programs, does not cover aducanumab. There are no known drug-drug interactions, so patients may continue memantine, cholinesterase inhibitors, and psychotropic drugs as indicated.
Aducanumab in Alzheimer Disease Research Review and Resources
Aducanumab is recommended only for patients with a formal diagnosis of mild cognitive impairment or mild Alzheimer disease, as determined by a thorough history, neurological examination, and objective cognitive assessment. Experts recommend a Mini Mental Status (MMSE) score cut off of at least 21, or an equivalent score on other validated measures (e.g., 17 or greater on the Montreal Cognitive Assessment (MoCA)). Clinical evaluation should focus on ruling out the presence of other non-Alzheimer disease pathology. This includes psychiatric conditions like uncontrolled depression or substance abuse, metabolic abnormalities like hypothyroidism or nutritional deficiency, and other neurologic conditions such as parkinsonism or rapidly progressive dementia.
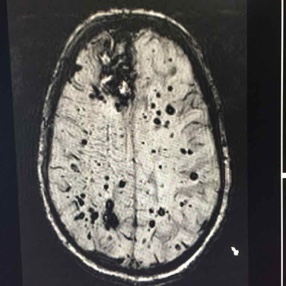
After clinical eligibility is established, the presence of cerebral amyloid must be confirmed through amyloid PET scan or cerebrospinal fluid analysis. An MRI should be done on all patients within one year of drug initiation to rule out the presence of microhemorrhages/hemosiderosis and non-Alzheimer disease pathology such as normal pressure hydrocephalus or vascular dementia. MRI should be repeated for monitoring prior to the fifth, seventh, and 12th doses, and as needed for any new or concerning symptoms. Some clinicians may also choose to screen prior to the 10th dose in higher risk patients (like those with APOE). At minimum, scans should include fluid attenuated inversion recovery (FLAIR), T2-weighted gradient echo (GRE), and diffusion-weighted imaging (DWI) sequences.
Amyloid-related imaging abnormalities (ARIA) is the most concerning side effect of aducanumab, and appears in a dose-dependent fashion. Patients and families should be vigilant about reporting new neurological symptoms because ARIA is typically reversible with medication discontinuation. The most common symptoms are mental status change, visual disturbance, dizziness, incoordination, and nausea. Untreated ARIA can progress to edema, microhemorrhages/hemosiderosis or, uncommonly, hemorrhagic stroke. Monitoring MRIs are needed in all patients because 75% of cases in the study population were asymptomatic at the time ARIA was identified.
Because of the increased risk of intracranial bleeding, aducanumab is contraindicated in patients with coagulopathy, including those on blood thinners, or with a history of hemorrhagic stroke. The APOE-4 allele, which is associated with development of late-onset Alzheimer disease, appears to confer a higher risk of ARIA with edema or hemorrhage. Genetic testing is not required but may be offered if the result is likely to influence patients’ decision about whether to use aducanumab.

Full Report
Read the full report from the American Academy of Neurology: Aducanumab Use in Symptomatic Alzheimer Disease Evidence in Focus

Alzheimer Disease Patient Cases
Click on the image to see the full case details and sign in to view community comments.
See more cases like these
Sign up for Figure 1 and gain access to a library of 100,000+ real-world cases.

Neurology Clinical Quiz
Take this quiz in the app to test your knowledge and view community comments.
ww Quick Quizw
What is causing this patient’s cognitive decline?
A 67-year-old presents with three years of progressive short-term memory loss. The forgetfulness has affected daily activities such that they are no longer able to go shopping or manage their finances on their own. On cognitive testing in the clinic, with the Montreal Cognitive Assessment, the patient scores …

Free CME
Clock Draw: Countdown to Dementia
View classic presentations of dementia-causing processes as they manifest in the clock-drawing test and recognize various domains of cognition that can be tested with a clock drawing test.
Start activity >
Free Neurology CME
Sign up for Figure 1 and gain access to 100,000+ opportunities for CME credits—valued at more than $2,000—for free.


References and Related Research
Aducanumab: Appropriate Use Recommendations | PubMed
“An Expert Panel was assembled to construct Appropriate Use Recommendations based on the participant populations, conduct of the pivotal trials of aducanumab, updated Prescribing Information, and expert consensus. Aducanumab is an amyloid-targeting monoclonal antibody delivered by monthly intravenous infusions. The pivotal trials included patients with early AD (mild cognitive impairment due to AD and mild AD dementia) who had confirmed brain amyloid using amyloid positron tomography. The Expert Panel recommends that use of aducanumab be restricted to this population in which efficacy and safety have been studied.”
Aducanumab Use in Symptomatic Alzheimer Disease Evidence in Focus: A Report of the AAN Guidelines Subcommittee | PubMed
“The author panel systematically reviewed available clinical trial data detailing aducanumab use in individuals with early symptomatic Alzheimer disease. Level of evidence statements were assigned in accordance with the American Academy of Neurology’s 2017 therapeutic classification of evidence scheme. Safety information, regulatory decisions, and clinical context were also reviewed.”

Related Medical Calculators
International Working Group (IWG) 2 Criteria for Alzheimer’s Disease Diagnosis
NINCDS-ADRDA Criteria for Alzheimer’s Disease
Montreal Cognitive Assessment (MoCA)

Kaci McCleary, MD
Hospice and Palliative Care Fellow, OhioHealth